A sound approach for effective gene therapy delivery to brain
Hong Chen’s lab develops noninvasive focused ultrasound intranasal delivery method
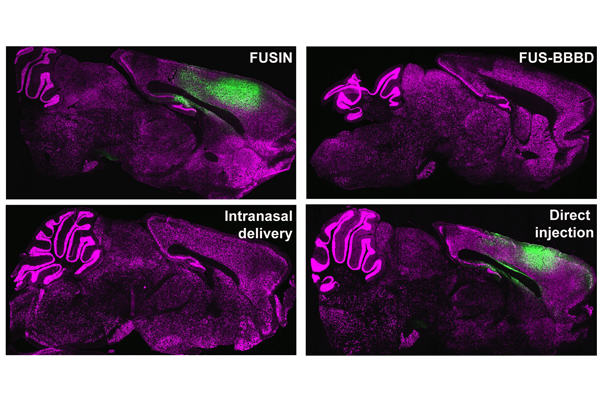
Researchers have been experimenting with different ways to deliver genes to the brain to treat central nervous system diseases and tumors. One of the obstacles, however, is the ability to penetrate the blood-brain barrier while having minimal effect on the other organs in the body.
Hong Chen, associate professor of biomedical engineering in the McKelvey School of Engineering and of radiation oncology in the School of Medicine at Washington University in St. Louis, and her team found an effective method to overcome that obstacle using focused ultrasound intranasal delivery (FUSIN). In new research, they found that the intranasally delivered gene therapy had comparable or better outcomes than existing methods while having minimal effect on the body’s other organs.
Results of the research, led by Chen and Dezhuang Ye, a postdoctoral research associate, and collaborators, are published online in eBioMedicine Sept. 21. It is the first study to evaluate the potential of FUSIN to deliver adeno-associated viral vectors, small viruses used to deliver gene therapy, in a mouse model.
Chen’s lab has used FUSIN for several years, finding promising results in a brain tumor model in 2021 and delivering gold nanoparticles in 2018. The delivery method uses the nasal route for direct nose-to-brain drug administration, bypassing the blood-brain barrier and minimizing exposure to the rest of the body. Microbubbles are injected into the blood and oscillate once they reach the ultrasound-targeted tissue. This bubble oscillation enhances the transportation and accumulation of intranasally administered drugs at the brainstem.
There are several existing methods of gene therapy delivery in humans: invasive direct injection into the brain; delivery through the nose; and an intravenous injection with focused ultrasound-induced blood-brain-barrier disruption, which is inefficient and includes a high risk of toxicity in the rest of the body.
In the new research, the team used adeno-associated viral vectors to deliver an enhanced green fluorescence protein to the cortex and the brainstem using focused ultrasound and microbubbles. They found that FUSIN achieved similar outcomes as direct injection, a more than 400-fold higher delivery efficiency than focused ultrasound-induced blood-brain-barrier disruption, and a more than 2,000-fold higher delivery efficiency than intranasal delivery alone. In addition, FUSIN had minimal effects on other organs in the body, with a small amount found in the lungs.
“Focused ultrasound uses ultrasound waves that can penetrate the scalp and skull to target virtually any location inside the brain, where it induces microbubble expansion and contraction that push and pull on the adjacent blood vessel wall,” Chen said. “The FUSIN procedure is entirely noninvasive and has successfully delivered multiple agents to different brain regions with high efficiency and minimal systemic exposure.”
To date, no gene therapies have been approved for diseases of the central nervous system. Chen said methods that can noninvasively deliver and spatially target adeno-associated viral vectors to the brain would have a significant therapeutic impact.
“We hypothesize that FUSIN-mediated adeno-associated viral vector delivery can achieve higher therapeutic efficacy than intranasally alone and potentially be used to treat various brain diseases, such as Parkinson’s disease,” Ye said. “However, future studies are needed.”
Ye D, Yuan J, Yang Y, Yue Y, Hu Z, Fadera S, Chen H. Incisionless targeted adeno-associated viral vector delivery to the brain by focused ultrasound-mediated intranasal administration. EBioMedicine, Sept. 21, 2022. https://doi.org/10.1016/j.ebiom.2022.104277
This research was supported by funding from the National Institutes of Health (R01EB027223, R01EB030102, R01MH116981, and UG3MH126861).