Reflecting on 20 years of progress in interfacial sciences and engineering
Young-Shin Jun and collaborators review interfacial reactions and look ahead to new frontiers in the field
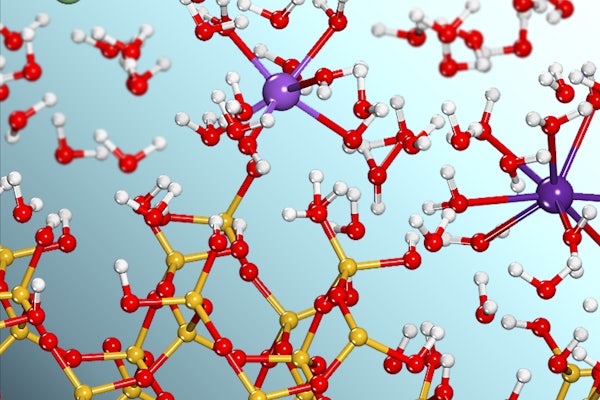
Interfacial reactions happen at the boundary where materials in different phases, for example a solid and a liquid, meet each other. These reactions drive all elemental cycling on Earth and play pivotal roles in human activities such as agriculture, water purification, energy production and storage, environmental contaminant remediation, and nuclear waste repository management.
In a review paper published May 24 in Chemical Reviews, 23 experts in solid-water interfaces celebrate the scientific advances in the field of interfacial reactions since the seminal paper on the topic was published in 1999. They also present new avenues and opportunities to explore in the decades ahead.
“It’s truly an honor to work with outstanding scholars from around the world to report 20 years of progress in interfacial sciences and engineering,” said Young-Shin Jun, professor of energy, environmental & chemical engineering in the McKelvey School of Engineering at Washington University in St. Louis and one of the leading and corresponding authors on the review paper. “Throughout this project, even during the dark time of the pandemic, many of us met virtually to work on the mega collaboration review paper, and we all remain hopeful and eager for the impact this science can have.”
The paper’s authors lay out the key advances in the 21st century that led to a more detailed understanding of mineral aqueous interfaces. Techniques that enable atomic- and nanometer-scale measurements helped uncover scale-dependent phenomena whose reaction thermodynamics, kinetics and pathways deviate from previous observations made on larger systems. New experimental evidence confirmed what scientists previously hypothesized but could not test, particularly how nontypical chemical structures drive interfacial chemical reactions. And, progress in computational chemistry yielded new insights that led to a molecular model of complex interfaces.
“Beyond looking at how far we’ve come in understanding realistic, rather than idealized, solid-water interfaces, the review captures new challenges, frontiers and opportunities in interfacial sciences,” Jun said. “We anticipate that the next 20 years will focus on expanding our understanding over greater spatial and temporal ranges as well as in systems of greater structural and chemical complexity. I’m excited to work with collaborators across disciplines and continents to achieve this great aspiration.”
Bañuelos JL, Borguet EU, Brown GE Jr., Cygan RT, DeYoreo JJ, Dove PM, Gaigeot M-P, Geiger FM, Gibbs JM, Grassian VH, Ilgen AG, Jun Y-S, Kabengi N, Katz L, Kubicki JD, Lützenkirchen J, Putnis C, Remsing R, Rosso KM, Rother G, Sulpizi M, Villalobos M, Zhang H. Oxide- and Silicate-Water Interfaces and Their Roles in Technology and the Environment. Chemical Reviews, May 24, 2023. DOI: https://doi.org/10.1021/acs.chemrev.2c00130
Jun received financial support from U.S. National Science Foundation’s Environmental Chemical Sciences program (CHE-1905077), the American Chemical Society’s Petroleum Research Fund (62756-ND5), and the U.S. Department of Energy Office of Science (DE-SC0023390). Jun and the rest of the authors also thank James Ballard, senior lecturer and director emeritus in McKelvey Engineering’s Division of Engineering Education, for his editorial support and careful review of the entire article.